Hard water affects over 60% of UK homes, leading to limescale buildup in appliances and fixtures, especially those that heat up, like kettles and washing machines.
Discover various methods to tackle limescale deposits caused by hard water and learn about their effectiveness below.

Limescale deposits around tap caused by hard water
What Causes Hard Water?
In the UK and worldwide, mains water often comes from storage reservoirs or underground wells and springs.
Reservoir water typically originates from rain or snow, flowing through rivers and streams before being collected.
As water travels through materials like chalk, gypsum, and limestone, it picks up minerals. The primary minerals causing hard water are magnesium and calcium.
These minerals remain in the water, entering homes and depositing on surfaces and appliances, leading to limescale buildup.

Water running through limestone collects minerals and causes hard water
Understanding Temporary and Permanent Hard Water
Hard water can be classified into two main types: permanent and temporary.
Permanent hard water, also known as “non-carbonate hard water”, poses fewer issues as it doesn’t cause limescale buildup in appliances and plumbing systems. This is because the sulphate ions dissolved in the water remain unchanged even when heated.
On the other hand, temporary hard water, also known as “carbonate hard water” is more problematic. It contains magnesium and calcium carbonates, which are responsible for limescale formation.
When hydrogen carbonate ions in the water convert to carbonate ions, they react with calcium and magnesium to produce calcium and magnesium carbonate, commonly known as scale, especially when the water is heated.
The Impact of Hard Water and Limescale: What You Need to Know
Hard water poses numerous challenges in households, primarily damaging household appliances that rely on water, such as dishwashers, washing machines, and kettles.
Over time, dissolved mineral deposits from hard water settle on internal components of appliances that come into contact with water. Heating elements and other hot surfaces are particularly susceptible to limescale buildup, which can impair their efficiency and longevity.

Heating element in kettle covered in limescale deposits
Additionally, heating systems and boilers are at risk from limescale accumulation. Deposits can insulate heating elements, making boilers less efficient and increasing energy costs. Furthermore, limescale can obstruct pipes and radiators, reducing water flow and further impacting efficiency.
Even a thin layer of limescale (1mm) can decrease efficiency by over 7%, significantly affecting heating costs.
Limescale buildup isn’t limited to appliances and heating systems; it can also affect tap spouts, shower heads, and areas prone to water leaks.
Hard water can leave spotty deposits and clouding on glass surfaces, like shower enclosures and glassware cleaned in dishwashers.

Misting on glass in the dishwasher caused by hard water – Image courtesy of simplelifeandhome.com
Clothes washed in hard water may feel stiff and scratchy after drying, while towels can appear lacklustre.
Another significant issue is the impact of limescale on cleaning agents such as soap, shampoo, and detergents. Calcium and magnesium deposits in hard water react with soap, forming “soapy scum” or soap curd, which is ineffective and leaves residues on skin and surfaces.

Soap scum deposits left around bath caused by hard water – Image courtesy of Pinterest
Hard water can also impact skin and hair health, causing dryness, frizziness, and dullness. Soap scum deposits on skin can clog pores and lead to skin conditions like dermatitis and eczema.
Addressing hard water issues is crucial to maintaining appliance efficiency, reducing energy costs, and preserving the quality of household items and personal hygiene.
Is Limescale and Hard Water Bad for Your Health?
While hard water and limescale can significantly damage household appliances over time, concerns about their impact on human health are often overstated.
Hard water may cause some irritation to hair and skin, but it is not considered a health hazard.
In fact, some experts view hard water as beneficial because it adds magnesium and calcium to diets, particularly in areas where water is very hard. This can contribute significantly to a person’s daily intake of these minerals.
Where are the Hard Water Areas in the UK?
Several regions in the UK are known for their hard water due to the geological composition. Areas in the South and East, rich in chalk and limestone, have particularly hard water quality.
In contrast, the North and West regions, which are predominantly composed of granite and sandstone, tend to have softer water.
For a visual representation, refer to the map below which outlines the distribution of soft, medium, and hard water areas across the UK.
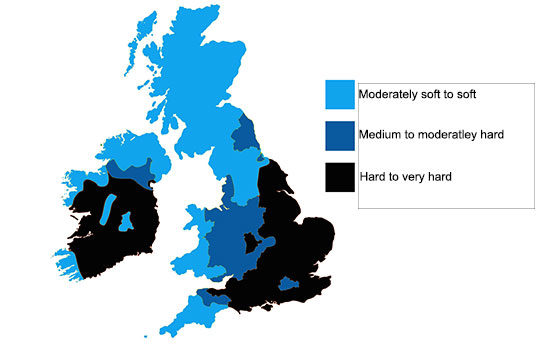
Hard and soft water locations around the UK
How to Treat Hard Water at Home
While hard water isn’t harmful to your health, it can cause limescale deposits and soap scum, requiring frequent cleaning. If you’re looking to soften your water and reduce these issues, consider the following solutions:
Water Softening and Conditioning Systems
When it comes to treating hard water, there are two primary methods: water softening and water conditioning. Each method has its own set of advantages and disadvantages.
Water Softeners
Water softeners operate on an ion exchange principle, where they remove calcium and magnesium from water and replace it with sodium chloride, commonly known as salt.
A typical water softener connects to the incoming mains water pipe. The untreated water passes through negatively charged beads, which attract and remove the positively charged minerals while simultaneously introducing sodium ions.
The treated water collects at the bottom of the storage tank where the process occurs and then flows throughout the house via pipes.
This method effectively softens the water permanently but has drawbacks such as high water and salt consumption, as well as substantial initial and operational costs.

Water softening system
Water Conditioners
Water conditioners operate differently from water softeners by preventing mineral deposits in hard water from solidifying on surfaces, rather than replacing elements in the water. They use various techniques such as magnetism, electrolytics, and electrical currents to achieve this.
For example, the Eddy water descaler is an electrical current-based water conditioner.

Electrical water conditioning system by Eddy
To find out more about the Eddy water softener, including how to install it, see our project here.
Using White Vinegar for Cleaning
Distilled white vinegar is a versatile household cleaner effective on various surfaces for removing dirt, grease, and limescale buildup.
Its acidic nature dissolves calcium-based limescale and effectively tackles soap scum. However, it should not be added to heating systems or used in water.
For cleaning purposes, white vinegar is a potent solution that requires some manual effort to achieve optimal results.

Distilled white vinegar is great for removing limescale and soap scum
Reducing Mineral Deposits
The temperature of hard water significantly affects how quickly mineral deposits, like limescale, accumulate. Higher temperatures accelerate a process called “mineral precipitation”, where dissolved minerals form deposits.
Lowering water temperatures slows this process, reducing limescale buildup.

Turn your boiler temperature down to slow mineral precipitation
Appliance Cleaning and Maintenance
To maintain efficient operation and prevent limescale buildup in appliances and heating systems over time, regular cleaning of internal components and related pipework is crucial.
Vinegar, combined with water and baking soda, is highly effective in removing limescale from various appliances such as washing machines and dishwashers. Detailed guides on cleaning appliances with vinegar can be found here.

Cleaning a washing machine with white vinegar solution
For dishwashers, ensure the salt container remains filled to soften the water effectively.
Alternatively, specific products like Calgon are designed for washing machines to combat limescale buildup. More information about Calgon can be found here.
Choose detergents and washing powders formulated for the hardness of water in your area, indicated on packaging or the manufacturer’s website, to optimise cleaning performance.
To prevent damage from hard water and limescale buildup in your home, consider installing a water softener or conditioner. Regularly use vinegar or other cleaning solutions to reduce mineral deposits and maintain the efficiency of your appliances and heating systems.

